Date: September 2020
生物发光成像(BLI)是一种非侵入式光学成像方法,旨在实现组织中生物发光信号的可视化和量化。1 BLI的根本是当酶作为分子报告基因在体内表达时,对底物的酶介导氧化过程中产生的可见光进行检测。
与使用终端终点、侵入性操作或放射性标记的传统成像和生物分布研究相比,BLI提供了可靠、灵敏和高通量的替代方案。 We were the first CRO to offer BLI in 2003 and in the 17 years since we have amassed considerable experience and know-how in this optical imaging field. 在此技术聚焦中,我们将介绍生物发光成像的原理,并重点介绍该临床前服务在癌症检测、监测疾病进展和体内抗肿瘤功效评估方面所提供的优势。
BLI要求细胞表达萤光素酶,该词源自拉丁语lucifer——发光物质,例如由萤火虫或海肾产生的发光物质。萤火虫萤光素酶基因于19852年初次实现克隆,自那时起,绿色最常用于荧光色成像。萤火虫萤光素酶需要注入其底物D-萤光素,该荧光素会产生在562 nm时达到峰值的生物发光信号,该信号由放置在不透光柜中的高光子转换效率电荷耦合器件相机(CCD)捕获。 The sequence of events leading from engineering tumor cells to express luciferase to imaging animals in vivo is illustrated in Figure 1. We use IVIS® In Vivo Imaging Systems (PerkinElmer, Waltham, MA) which allow high sensitivity and high resolution in vivo BLI and fluorescence imaging (FLI) across a wide range of wavelengths. 在2%异氟烷气体麻醉下,一次最多可对五个动物进行成像。为每只小鼠注射D-萤光素,并在注射后10-15分钟内对小鼠进行成像。BLI信号在肿瘤、特定区域(例如颅骨、胸腔或腹部)、整个身体或离体组织周围绘制的感兴趣区域(ROI)中进行量化,并且该信号按照光子/秒表达,代表来自用户定义区域的全向辐射通量。图像使用Living Image 4.3.1(PerkinElmer,马萨诸塞州沃尔瑟姆)软件分析。
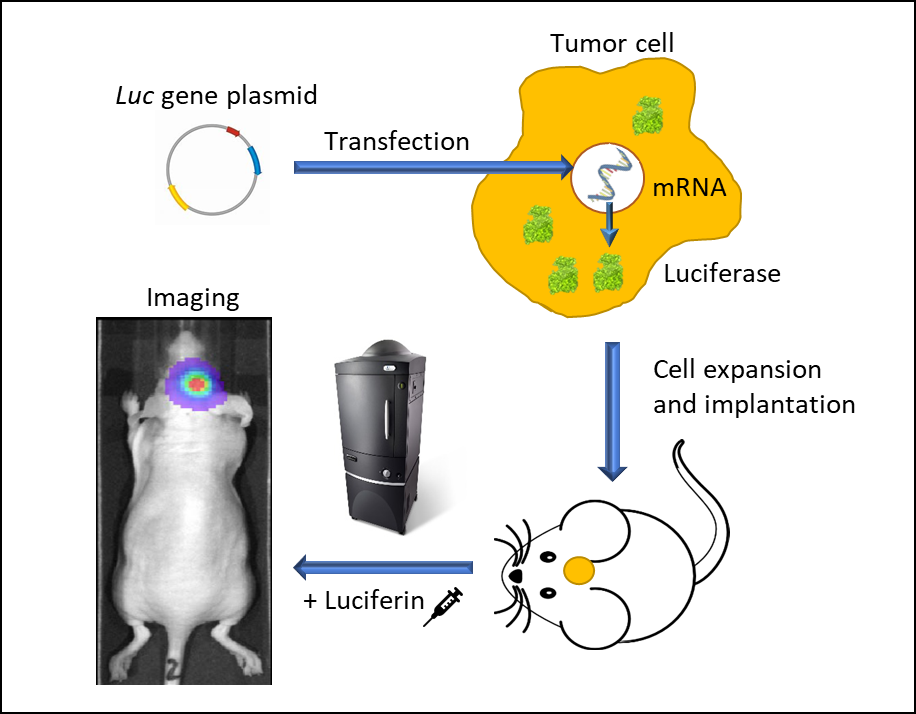
图1:使用发光活细胞的体内生物发光成像。将携带有启动子驱动荧光素酶基因的载体转染到肿瘤细胞中。稳定转染的细胞扩增并植入小鼠体内。肿瘤生长后,给动物注射荧光素并在麻醉下在IVIS成像系统中成像。荧光素酶细胞会发光,并可进行时空跟踪。
监测原位实体瘤生长
过去20年里,凭借出色的有效性和敏感性,BLI方法和工具的应用越来越广泛,包括:蛋白质间相互作用、基因筛选、细胞周期调节剂、寄生虫感染、干细胞植入跟踪、NK细胞迁移以及临床前肿瘤学。大量研究表明,BLI成像可以暂时评估肿瘤的发展。3当肿瘤生长无法通过卡尺测量确定,并且依赖于体重减轻等临床体征或动物安乐死后的肿瘤负荷评估时,这一点尤为重要。在下述示例中,所有动物护理和使用均根据动物福利法规在AAALAC认可的研究中心进行,并经过IACUC协议审查和批准。
图2中的实例突出了非侵入性BLI在监测深层组织肿瘤发展方面的价值。将NCI-H460-Luc2人类肺癌异种移植物原位植入(OT)(1x105细胞/小鼠)雌性裸鼠的左肺。BLI(图2,左上)显示了最早在肿瘤细胞植入后8天就可以检测到的胸腔肿瘤(因在第28天可查看的最大信号阈值而在图像中不可见),而且肿瘤负荷在接下来的2周内增加。在整个研究过程中,小鼠的平均体重减轻百分比为18.3%,可能与疾病有关(未显示),并且小鼠在肿瘤细胞植入后28天安乐死。尸检揭示了原发性肿瘤的明显生长以及整个胸腔肿块的实质性发展。在图2(左下)中,雌性白化病C57BL/6小鼠颅内植入GL261-luc2同系鼠神经胶质瘤细胞(1x106细胞/小鼠)。 植入后7天,大脑中可检测到肿瘤。与疾病进展相关的体重减轻在该模型中很常见,并且在植入肿瘤后11天就可以检测到,而死亡中位时间约为21天。在雌性裸鼠中进行心内注射后,人类乳腺腺癌细胞系MDA-MB-231-luc-D3H2LN形成骨病变(1x105细胞/小鼠)。在注射肿瘤细胞21天后,骨病变清晰可见,并且在接下来的3周内,肿瘤数量和肿瘤总负荷都有所增加(图2,右侧,顶部和底部)。到第32天,小鼠体重最多减轻16.2%。体重减轻很大程度上与模型的侵袭性有关(未显示)。这些研究的主要启示是,BLI可成为一种强大的工具,用于实时、无创地研究各种类型的肿瘤,而不必完全依赖于可能会或可能不会伴随肿瘤负荷增加的疾病临床症状。
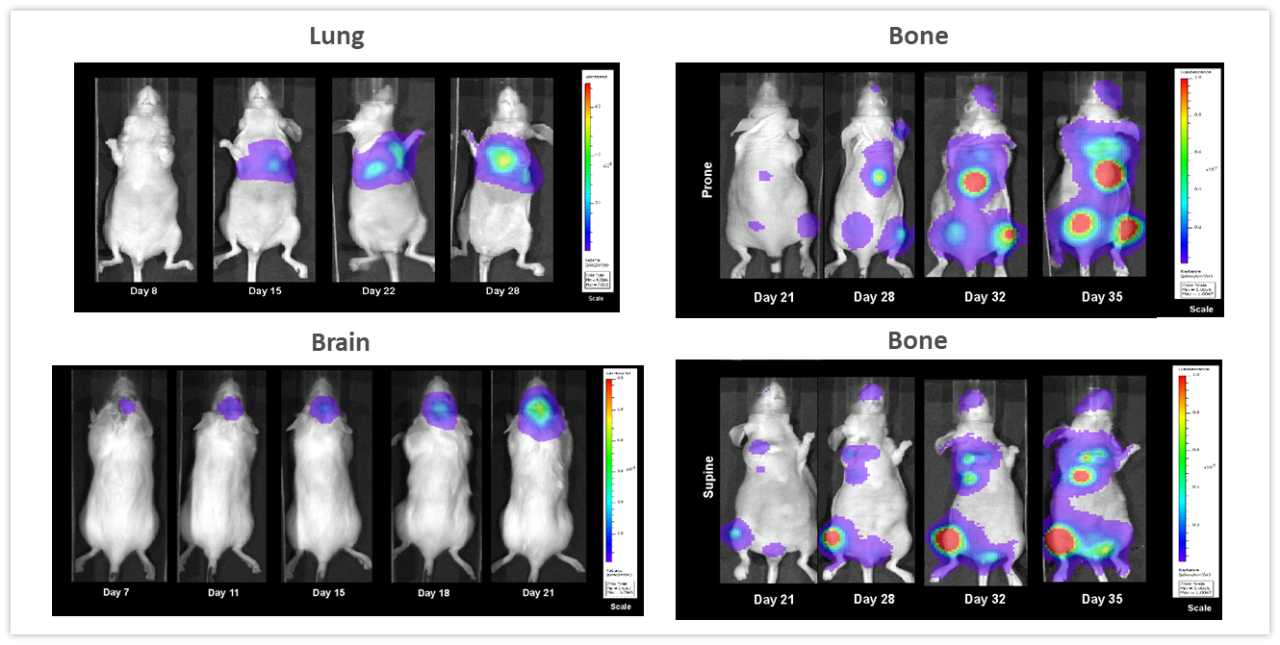
图2:多种体内模型的代表性BLI图像。左上:在雌性裸鼠OT(左肺)中植入的NCI-H460-Luc2人肺癌。左下:在雌性白化病C57BL/6小鼠中植入的同基因鼠神经胶质瘤GL261-luc2细胞,右:雌性裸鼠心内注射后人乳腺肿瘤MDA-MB-231-luc-D3H2LN骨病变,显示俯卧(上)和仰卧(下)位置的全身图像。
血液恶性肿瘤成像
With over 80 unique hematological malignancy cell lines, we lead the industry with market-relevant hematologic malignancy models, particularly pertinent in the adoptive cell therapy (ACT), also known as cellular immunotherapy, field.4 BLI is critically valuable when evaluating progression and treatment response in hematological disseminated malignancies. 在4种经静脉内注射到NSG小鼠中的不同人类血液癌细胞中进行的纵向研究显示,在所有模型中,肿瘤负荷随时间的推移都会增加(图3),凸显了BLI在重复评估疾病进展和严重程度,以及了解整个人体中肿瘤信号的生物分布方面的价值。
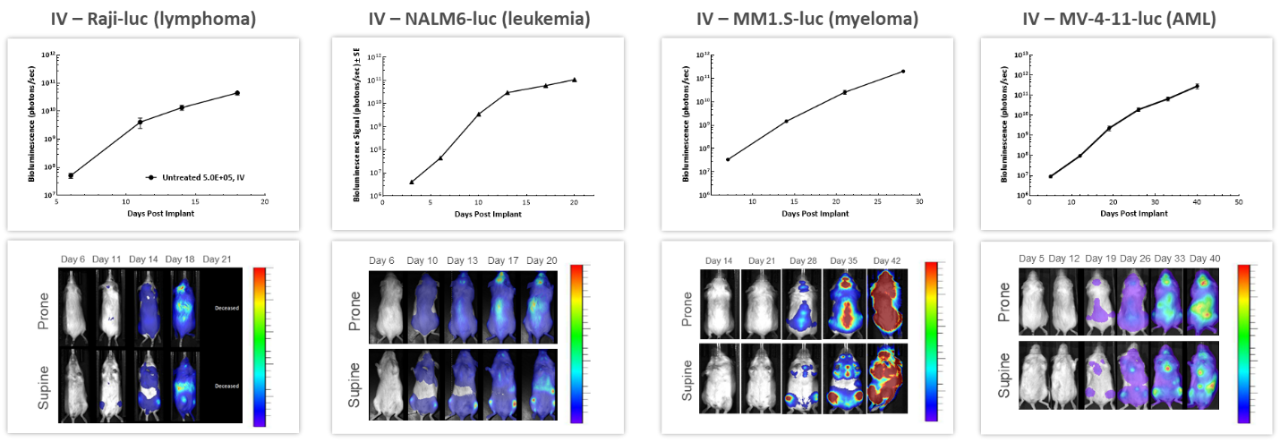
图3:在NSG小鼠中进行静脉注射后,若干体内人类血液系统恶性肿瘤的代表性BLI数据。
ACT不断发展,为患者提供更好、更个性化的方案。CAR-T细胞是肿瘤细胞表达的自体或异体T细胞特异性靶向抗原或标记物。在将这些新疗法应用于临床之前,对CAR-T细胞的疗效和安全性进行临床前体内评估至关重要。图4所示的研究旨在测试在NSG小鼠中经静脉植入的表达萤光素酶的Raji人B细胞淋巴瘤细胞中的不同CAR-T制剂和剂量。3种不同的CAR-T构建体显著抑制了肿瘤负荷并延长了生存期,突显了BLI为这些新型细胞免疫疗法的研发带来的价值。
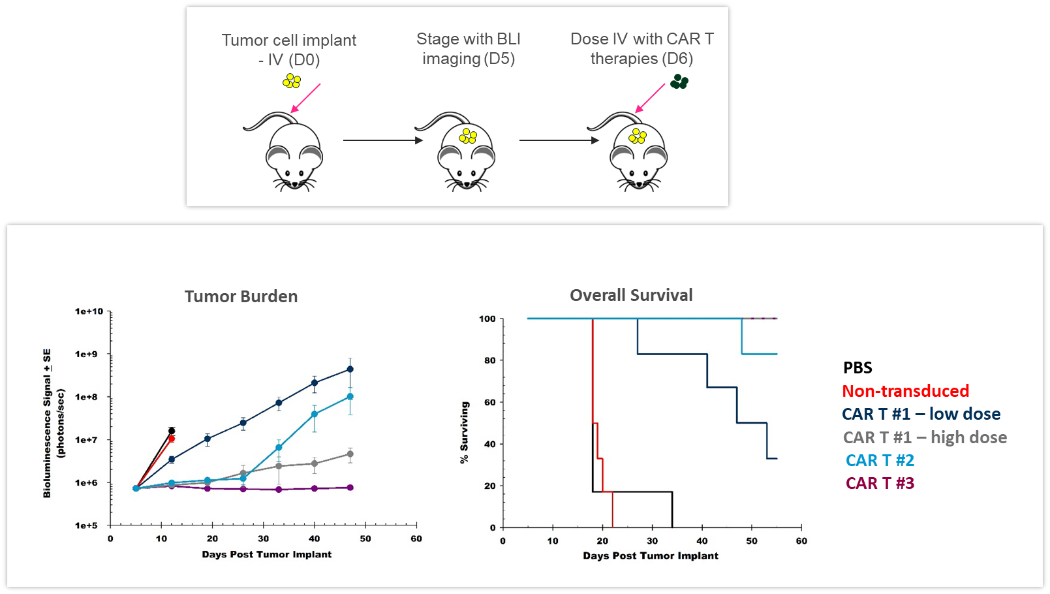
图4:CAR-T疗法对经静脉植入的表达萤光素酶的Raji人B细胞淋巴瘤的效果。上:肿瘤细胞接种和CAR-T治疗的示意图。下:通过BLI评估的肿瘤负担和整体生存期。
监测疗效
放射
显然,BLI的主要优势之一是能够随着时间的推移跟踪同一只动物的抗肿瘤治疗效果,从而减少了所使用动物的数量。在图5所示的示例中,雌性裸鼠颅内植入NCI-H1975-luc人非小细胞肺癌,以模拟肺向大脑的转移。小鼠接受了RadSource RS-2000辐照器两个疗程的分次放射治疗(2Gy;放疗5日,停止两日,共两个周期)。对照组接受假辐照。BLI图像记录了随着时间推移的情况,并证明放射线显著降低了肿瘤负荷(图5,在第19天p<0.05)并将寿命延长了160%(p<0.05,未显示)。在此实验中,我们能够量化体内治疗反应,这在监测疗效以及设计和完善新型疗法时至关重要。
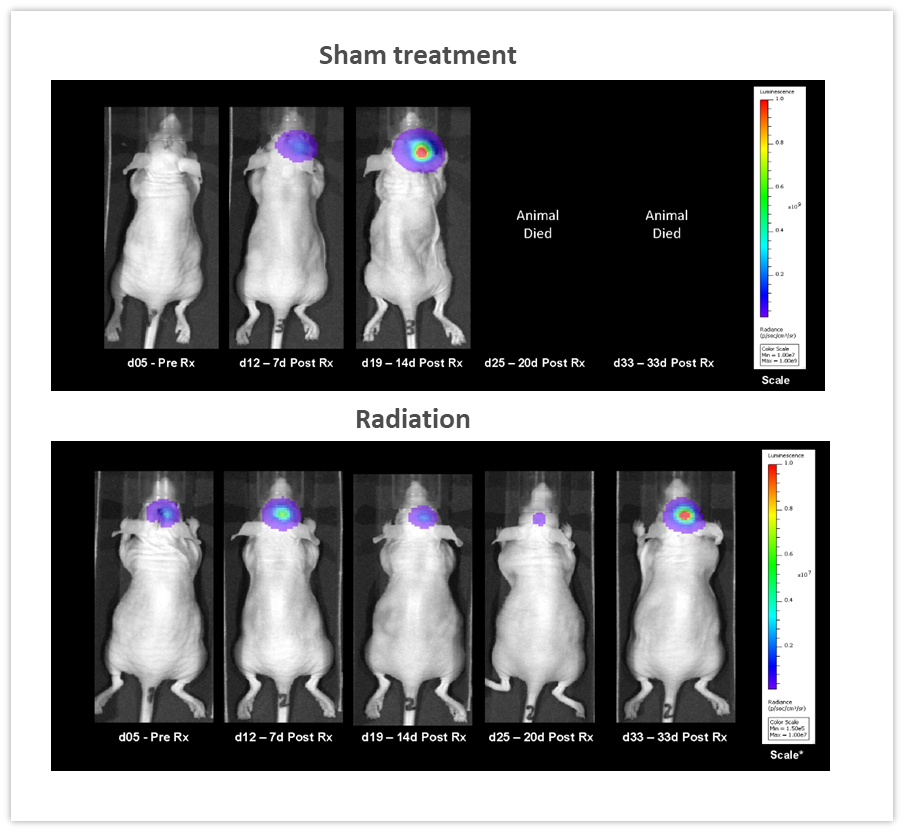
图5:针对颅内植入NCI-H1975-luc人非小细胞肺癌的雌性裸鼠的局部放射治疗(2Gy;放疗5日,停止两日,共两个周期)。
检查点抑制和联合治疗
在雌性白化病C57BL/6小鼠中颅内植入的鼠同基因GL261-luc2胶质瘤细胞中测试检查点抑制剂单药或与放疗进行联合治疗的疗效。小鼠未经治疗、采用小型动物放射研究平台(SARRP;Xstrahl Inc.)以单次7.5Gy剂量进行放射治疗、抗小鼠PD-1(克隆RMP1-14,10 mg/kg)治疗或由两种疗法进行联合治疗。如图6(左)所示,BLI信号在肿瘤细胞植入后6天已经可以检测到,并在接下来的几周中取得了进展。对产生的生物发光信号进行定量测量(右)表明了治疗对肿瘤负荷的影响。抗mPD-1治疗导致2.6天的肿瘤生长延迟和1/8的无肿瘤幸存者(TFS),而放疗导致16.6天的肿瘤生长延迟和2/8的无肿瘤幸存者。放疗和抗mPD-1联合治疗的结合导致可观的38.6天肿瘤生长延迟,7/8的小鼠完全缓解。测量位于体内深处恶性肿瘤中的抗肿瘤反应的传统方法是以末期为基础的,而且取得测量数据的过程通常十分耗时。如这些示例所示,整合体内BLI成像使我们从肿瘤治疗的“黑盒”转向个体反应的实时评估,从而更快地灵活调整和完善治疗方法。
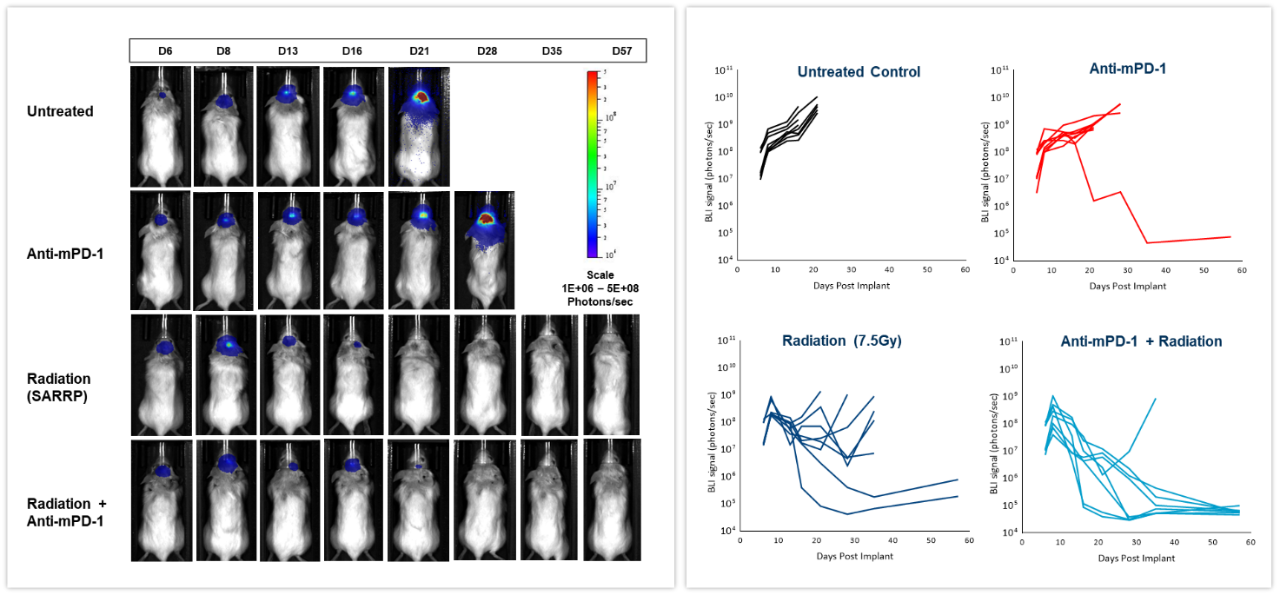
图6:针对雌性C57BL/6白化病小鼠颅内GL261-luc肿瘤的局部放射、抗mPD-1和联合治疗的效果。
多模态
BLI可以结合其他成像方法,以研究不同的生物学途径。为说明此应用,我们将同基因4T1-luc2-1A4小鼠乳腺癌细胞原位注入具有免疫活性的BALB/c小鼠的乳腺脂肪垫中。当肿瘤达到~300 mm3时,经静脉注射近红外显像剂ProSenseTM750以检测与侵袭性乳腺癌生长相关的组织蛋白酶活性。ProSenseTM750是一种光学沉默制剂,在用组织蛋白酶裂解后会发出荧光。如图7所示,在肿瘤中很容易检测到BLI和FLI肿瘤信号。但是,BLI和FLI信号并非完全重叠,这在我们的预料之中,因为所有肿瘤活细胞均会产生萤光素酶,而不仅肿瘤细胞会发出荧光信号,负责组织蛋白酶ProSenseTM750探针裂解和吸收的其他肿瘤相关细胞(主要是巨噬细胞)也会发出荧光信号。 Multimodality can thus be used to interrogate different biologies in vivo, simultaneously.
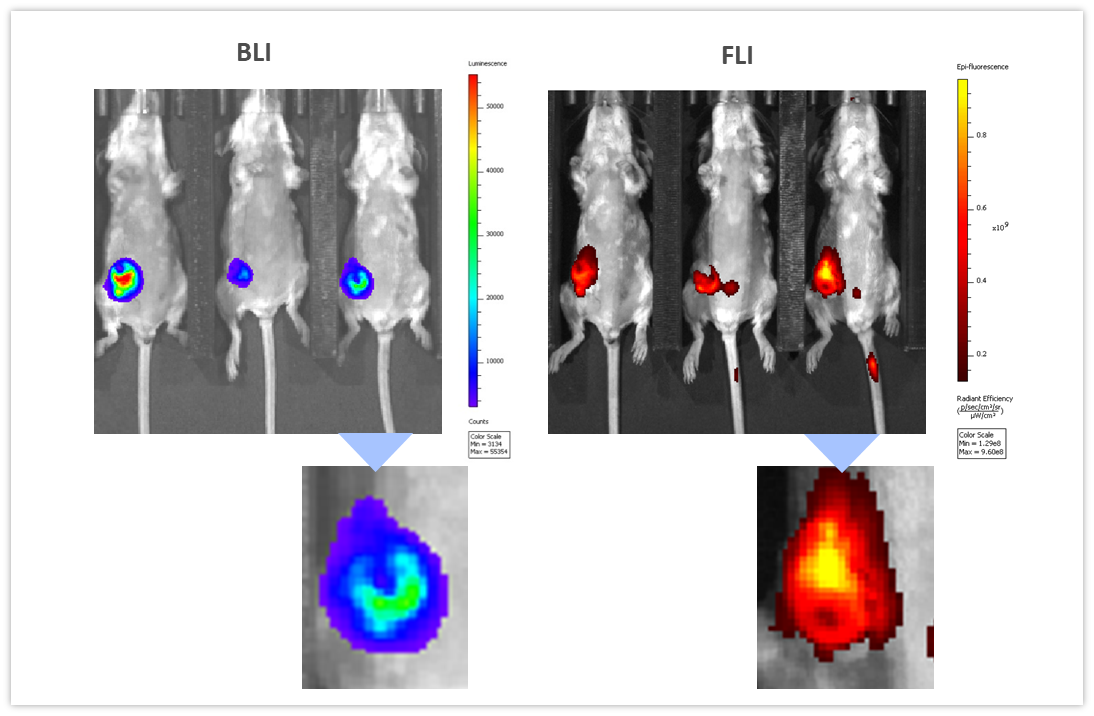
图7:静脉注射组织蛋白酶特异性ProSenseTM750荧光探针24小时后,BLI(左)和FLI(右)对4T1-luc2-1A4肿瘤的多模态成像。以下是相应肿瘤的特写。
总结
深层组织癌和癌症转移的小鼠模型主要依靠终末评估以评估肿瘤负荷,例如组织重量、结节数和/或组织学分析。自20多年前问世以来,BLI已经成为跟踪生物发光标记癌细胞系随时间推移的生长动力学以及对各种体内治疗方法的反应的重要技术,并可尽量减少研究使用的动物数量,因为可以随时间推移对同一对象进行成像,而无需进行终末评估。除了为使用成熟的侵入性或放射性同位素依赖性非侵入性成像方式提供替代方法外,BLI还为各种科学领域做出了重大贡献。
Contact the scientists to request the full data set or to learn more about our BLI service and how it can be applied to your preclinical research.
参考资料
1Contag CH、Spilman SD、Contag PR、Oshiro M、Eames B、Dennery P、Stevenson DK、Benaron DA。使用生物发光报告基因实现活体哺乳动物基因表达的可视化。《Photochem Photobiol》 1997;66: 523-531。
2de Wet JR、Wood KV、Helinski DR、DeLuca M。萤火虫荧光素酶cDNA的克隆及活性荧光素酶在大肠杆菌中的表达。《Proc. Natl. Acad. Sci. U.S.A.》1985;82, 7870-7873。
3 Jenkins DE、Oei Y、Hornig YS、Yu SF、Dusich J、Purchio T、Contag PR。生物发光成像(BLI)可以改善和完善传统的鼠类肿瘤生长和转移模型。《Clin Exp Metastasis》,2003;20(8):733-744。
4 https://www.cancerresearch.org/immunotherapy/treatment-types/adoptive-cell-therapy
让我们开始对话
联系我们